Mastering FDA Inspection Readiness: Key Strategies for Compliance Success
Inspection readiness is always a hot topic within the life sciences industry. Much attention is paid to ensuring that SOPs are in place, employees are
What Are the FDA’s Thoughts on Quality Culture?
Quality culture, a term that has been gaining traction in regulatory compliance, is taking center stage in the life sciences industry. In a recent webinar
The Costly Consequences of Adulterated Drugs: A Call for Stronger Regulatory Enforcement
In today’s pharmaceutical manufacturing landscape, trust is paramount. Patients rely on drug manufacturers' integrity to provide safe and effective medications to manage complex, targeted health
Navigating the Modernization of Cosmetics Regulation Act of 2022: A Decade of Evolution and Industry Impact
New requirements for cosmetic product manufacturers under the Modernization of Cosmetics Regulation Act (MoCRA) of 2022 are being phased in throughout 2024, affecting cosmetics products
Welcoming Diluks De Silva to The Compliance Architects® Team
We are thrilled to announce the newest addition to the Compliance Architects® family, Mr. Diluks De Silva, who joins us as an Executive Consultant. With
Achieve Excellence with CAPA: Your Key to Compliance Success
In the realm of regulatory compliance, there's one acronym that consistently looms large: CAPA or Corrective and Preventive Action. It's a term that resonates with
Understanding and Implementing Potency Assurance Strategies for Cellular and Gene Therapy Products
In the dynamic landscape of biopharmaceuticals, ensuring the potency of cellular therapy or gene therapy (CGT) products is a paramount objective. This draft guidance serves
Data Integrity: The Foundation of Medical Product Manufacturing Compliance
The FDA and other global regulators rely on data reviews during inspections to determine whether a pharmaceutical or medical device manufacturer operates within regulatory requirements and expectations. The credibility
Navigating the Future of CAR T Cell Therapy Development: Insights from FDA Guidance
Cell and gene therapies represent a new medical frontier, offering hope for treating rare, orphan, and challenging diseases. However, the journey from development to market
Expert Tips on How To Prepare For an FDA Pre-Approval Inspection (PAI)
In the realm of quality and compliance within pharmaceuticals, biopharmaceuticals, and combination products, I’ve frequently been asked about the crucial steps to pass an FDA
Exclusive Insight: How FDA Reshapes Medical Device Quality Guidelines with ISO 31485-2016
The highly anticipated FDA Decision on the Harmonization of the Medical Device Quality System Regulations with ISO 31485-2016 has been released, marking a significant development
Proud to be a Silver Sponsor of RIC’s Inaugural Annual Meeting! Join us March 22-23, 2023 at Convene in Arlington, VA!
Compliance Architects is proud to be Silver Sponsor of this year's RIC's inaugural annual meeting! The REMS Industry Consortium fosters collaboration and innovation to advance
Talk to us about current talent trends, and what the industry will need in the next 5 to 10 years?
Transcript taken from 2022 PMWS by Executive Platforms The talent crisis as it has been called is certainly not something that has gone away or
Essentials of Post-Inspection Actions: From FDA Notices to Criminal Prosecution | August 18, 2022 | 2:00–3:30 PM ET | Live Webinar | FDLI
Attention life science quality and compliance professionals. Mark your calendars for this Thursday, August 18th at 2PM ET! Our colleague Teresa Gorecki is presenting with four
Randall “Randy” Lumia, SPHR, SHRM-SCP joins Compliance Architects® full-time as Vice President of Human Resources & Organizational Effectiveness
It gives us great pleasure to announce that Randy has joined us as a full-time Vice President of Human Resources & Organizational Effectiveness. As many of
What Does it Mean to Operationalize the Quality Agreement? – Taken from PMWS 2022
This concept of “Operationalizing the Quality Agreement” comes from my background. I'm an attorney in addition to being an engineer. I've done a lot of
John (Jack) Garvey Has Been Selected by Our Partners QbDVision to Join Their Newly Formed Board of Advisors
Taken from LinkedIn... "At QbDVision, we firmly believe that finding the right guides is just as important as discovering the right path. We’re incredibly fortunate
What Should Companies Concentrate On When Partnering with CMOs and CDMOs?
The CDMO and CMO relationships and the whole kind of business dynamic is critically important to the life sciences space. And particularly now with what
How Does Compliance Architects® Help the Biopharmaceutical Industry Achieve Its Goals?
I think the fundamental principle for all regulated industry whether it be biopharmaceuticals, medical devices, is that scientific concepts do not result in products for
How Has Risk Changed for the Biopharmaceutical Industry in Light of Recent Events?
Risk is still an important issue, and the fundamental premise is that there is so much to do. The science is so complex. The regulatory
What Are Some Of The Common Issues and Trends You Are Hearing From Biopharmaceutical Executives in 2022?
What are some of the common issues and trends you are hearing from biopharmaceutical executives? There were trends that were occurring before the COIVID crisis,
How Does Quality Pulse® Align with ICH Q10
Gorecki: Very well. Ken, do you want to take that first? Ray: Sure, I’ll take a stab at it. If you look at Q12 and
Visualizing Quality Culture Data – Analyzing Two Quality Culture Scenarios
Now let’s look at a third quality culture scenario, where you have a weak or ambiguous culture. Notice in this one that expressed values is
Quality Culture Scenarios – Good vs. Bad
It’s our belief that culture poses a direct risk to life science companies. And by understanding your culture and having a predictive diagnostic model that
The Scientific Basis of Quality Pulse®
Edgar Schein was a researcher in the 1950s and ‘60s in organizational dynamics and came up with a model that included what the organization says
A Low Cost, Seven-Step Approach to Reducing FDA Enforcement Risk
August: normally full of summer… sun, sand and surf. As the song goes, “summertime and the living is easy…” In 2020, COVID-19 — and more
A New Quality Culture Paradigm
These two slides date back almost 20 years, and the FDA intuitively saw that culture has a tremendous impact on the outcomes of life science
The Winchester House of Quality
Well designed and executed quality systems are the foundation of compliance for the highly regulated pharmaceutical and medical device industries. Still, many companies struggle to
Compliance Architects® and CherryCircle Software Announce New Partnership to Drive Best-in-Class Drug Development
Compliance Architects® is pleased to announce that it has partnered with CherryCircle Software to support implementation and on-going support needs of CherryCircle’s clients. CherryCircle Software
What Makes Quality Pulse® Unique? How is it Different Than Other Tools on the Market?
Jerry Appert: Great, great discussion. When you think about all of the different applications and use cases that you both articulated so nicely, maybe one
What are the Technical Capabilities of Quality Pulse®?
Quality Pulse® has some very robust technical capabilities that are built in and accompany the research-based behavioral model that's behind the diagnostic. A couple of
How Does the Interactive / Visual Data Output of Quality Pulse® Help Executives?
Good executives make decisions based on facts and data. The Quality Pulse® output provides basically a high-level scorecard that is then drilled down into, and
Thoughts on the Biden Administration’s Search for a New FDA Commissioner: In the Words of Pink Floyd… “Is There Anybody Out There?”
It appears the Biden administration is having difficulty with selecting a new Commissioner of Food and Drugs (FDA Commissioner). (FiercePharma Story NBC News Story Endpoint News Story) I
How is Quality Pulse® Different Than Other Tools on the Market?
Quality Pulse® compares to other quality culture assessments in four key areas and differentiates itself quite well. First of all, Quality Pulse® is a research-based
What Are the Top 2-3 Skills That the Training & Learning Community Needs to Up-skill / Re-skill Their Staff to Support a Virtual Inspection or the Adaptation of the Audit Model?
Question: Thanks, Jack. That was informative. What would you consider to be the 2-3 top skills that the Training and Learning community needs to upskill
What Types of Companies Can Benefit from Quality Pulse®
The Quality Pulse® culture diagnostic tool is appropriate for all companies in biopharma and pharma, in regulated consumer products, in devices, and in combination products.
When Would a Company Consider Using the Quality Pulse® Culture Diagnostic? – Thoughts from our Senior Consultant, Ken Ray
Quality Pulse® has several use cases which it adds value to an organization. I’ll give 4 as an example, here. 1st occurs very often, a
What Does the Quality Pulse® Quality Culture Diagnostic Help Organizations Understand?
The Quality Pulse® diagnostic tool is a tool that helps an organization understand three things, really… How clearly messages regarding quality are shared across the
Blunt Talk from the FDA on Outsourcing Operations
I’ve read a lot of Warning Letters in my 30 years of working in FDA-regulated industry. But one issued this week made me stop and
Why is Measuring Quality Culture Important for FDA Regulated Industry?
Quality culture has been something that has been really hard to create tight, and kind of, geometric bounds around. But, in fact, it's something that's
FDA Inspections on Hold — But They’ll be Back. The Question is, Will You Be Ready? Highlights from FDLI’s Enforcement, Litigation, and Compliance Conference
Now that the dust has settled from FDLI’s Enforcement, Litigation, and Compliance Conference and everyone is back to the new normal (Zoom calls, casual clothes
Compliance Architects® Collaborates with Carmody Quality Solutions on New eBook, “The Science And Art of Building a VQA® Culture”
Carmody Quality Solutions, LLC (CQS), a consulting partner that provides quality management services to the pharmaceutical industry, announced a first-of-its-kind eBook that helps quality and
How Do You Measure Quality Culture in FDA-Regulated Industry?
Quality culture can be extremely difficult to measure quantitatively...especially for companies regulated by the FDA. In this video, CEO Jack Garvey explains how organizations can
What Should Quality Executives be Thinking About When it Comes to FDA Risk Management?
I love risk, and risk is something I live in every single day. I think it's really important to understand that when people talk about
Risky Reliance – Be Careful with FDA’s New Compliant-Manufacturing Resumption Guidance
Guidance Inartfully Mixes New and Old Expectations – Creates Confusion for Drug and Biologics Manufacturers As the COVID-19 pandemic continues, FDA continues its efforts to
How to Keep Pharma/Biotech/Device Manufacturing Facilities Open Using Expedited Change Control
For life science manufacturers concerned about third-party access to facilities, we have been recommending they add pre-entrance gowning to existing gowning procedures and extend the
6 Reasons to Work with an Interim Expert/Executive Placement Firm — Amazing How Executives Don’t Recognize the Risks in No. 4
1. The Opportunity Cost of Swapping Staff from One Project to Another The need to constantly swap internal resources to keep projects on track can
CBD Manufacturers Must Develop GMPs Even Absent FDA Instructions
Cannabidiol (CBD) is the latest “it” product. Companies across the US and internationally are eager to manufacture CBD or incorporate CBD into a variety of other
Risk Management and Data Integrity: Focusing on the Critical
The FDA has long taken a risk-based approach to manufacturer GMP compliance expectations and enforcement actions, as well as requiring pharmaceutical and medical device manufacturers
Systems, Controls, Challenges and Governance for Sustainable Data Integrity Outcomes
Being able to present data that the FDA believes it can trust is critical to making it through an inspection unscathed. Medical product manufacturers must
Is Your Data Getting You in Trouble? A Look at FDA Requirements and Expectations
Data drives the pharmaceutical and medical device industries. It informs the manufacture and release of medical products to patients and consumers, and determines which products
Consideration of ICH Guideline, Industry Norms Necessary for Compliant Clinical Quality Assurance Plan
Savvy companies already understand that Quality Assurance (QA) must be integrated into the clinical development system, not relegated to an afterthought of audits and quality
Investigations, Analyses and Conclusions: Finalizing Well-Written, Inspection-Ready Documents
Well-drafted, easy-to-understand quality records are crucial to helping pharmaceutical and medical device manufacturers navigate FDA inspection obstacles. The FDA relies heavily on information presented in
Dedicated, Integrated Quality Assurance Systems Critical to Successful Clinical Trials
The notion that the role of Quality Assurance (QA) in clinical development should be relegated to reviews, audits and quality control (QC) checks is an
AAMI Has Just Released an Important Document Mapping US 21CFR 820 to the Medical Device Standard ISO 13485
Are you a device or combination products manufacturer or service provider? AAMI has just released an important document mapping US 21CFR 820 to the Medical
How Quality Records and Documents Create Your Biggest FDA Inspection Risk
Risk management is an essential activity for regulatory compliance within pharmaceutical and medical device companies. While it is often challenging for medical product manufacturers to
Why Is There A Lack of Connection Between FDA Inspection Results and True Quality Outcomes?
After decades in the pharma, device, and biologics industry working in new product development quality; manufacturing quality; market quality and post market surveillance; and quality
Why Do FDA Inspections Often Turn Out Bad?
Why do good manufacturing operations have bad inspections? FDA warning letters and 483s are rife with references to manufacturing controls and batch failures; to SOPs
Writing: The FDA Compliance Problem No-One Talks About
There are no FDA regulations governing writing quality or clarity. FDA Investigators will never note “poor writing” as a 483 observation or warning letter citation. But
The Secrets We Keep — FDA Data Integrity, Elizabeth Holmes, Theranos, Bad Blood and HBO’s “The Inventor?”
By now, most everyone in FDA regulated industry is aware of Theranos, the Wall Street Journal’s John Carryrou, and his award-winning book Bad Blood: Secrets
FDA has Published a Guidance for Industry on Acceptance and Filing Review for PMA Applications
FDA has Published a Guidance for Industry on Acceptance and Filing Review for PMA Applications Key elements of this Guidance: - FDA has not changed
A Critical Guidance Has Been Issued – “Refuse to Accept Policy for 510(k)s”
Our very own Teresa Gorecki took to LinkedIn earlier this afternoon to announce... A critical Guidance has been issued by FDA; the new Guidance, “Refuse
New DRAFT FDA Guidance Published Today – Nonbinding Feedback
Important DRAFT FDA Guidance issued today (dated 2/19/19 however) regarding "non-binding feedback" after FDA inspections of certain medical device establishments. Limited to statutory (FDARA 704)
Seven Things to Consider When Selecting a Pharmaceutical Quality Consulting Firm
Selecting a pharmaceutical consultant to help your organization with GMP, quality and compliance concerns can be a daunting task for any Quality executive. This is
A Two-Data-Point Measure of Manufacturing Quality Culture
With my professional experience starting point as a plant-floor engineer, I’ve spent more than my fair share of time in pharmaceutical, medical device and other
Embracing “Quality” Over “Compliance” — A Misguided Approach to Improving FDA-Regulated Quality Outcomes
As someone with many years of experience working in and around FDA-regulated manufacturing operations, a recent LinkedIn post (March 2018) entitled “Why QUALITY Needs to
Akorn, Fresenius and Data Integrity – A Journey from ALCOA to Materiality and FDA Enforcement Risk
The following is a redacted transcript of a live, conference call question and answer session conducted on 16 March 2018 with a leading investment advisory
US DOJ Reverses Decades of Predictable Guidance to Life Sciences Industry
On January 25, 2018, the United Stated Department of Justice under Attorney General Jeff Sessions reversed decades of accepted regulatory agency practice by substantially reducing
Deputy Assistant US Attorney General Outlines New Approaches for Enforcement of FDA-Regulated Companies
On Thursday, December 7, Deputy Assistant Attorney General Ethan P. Davis, US Department of Justice, Consumer Protection Branch, gave a speech at the Food and
POPULAR SCIENCE; BAD JOURNALISM
Two months ago I posted in LinkedIn about a Popular Science article that unfairly characterized FDA’s regulatory process, and implied FDA tacitly agreed with a
Trump, Regulatory Reform & FDA Compliance: The Impact of the US Election on FDA Regulatory Enforcement
The election of Donald Trump as President-Elect of the United States was, and continues to be, a watershed moment in the 200+ year American experiment.
Quality Agreements – Cornerstone of Product Supply Relationships
I worked for a Chief Quality Officer a number of years ago who always had this response when our company was contemplating movement of a
FDA “Live Review” Inspection of Company Documentation: Are You Ready?
The following was included in our July email newsletter. There are many training sessions conducted each year by GMP Training Managers and company quality and
FDA Announces New Office of Dietary Supplement Programs (ODSP)
Opportunities and Risks for Dietary Supplement Companies for 2016 and Beyond 2015 is certainly ending with a bang. As many of us industry insiders have
Executive Insights from FDLI’s 2015 Enforcement, Litigation & Compliance Conference: Trends and Risks for 2016 and Beyond
Early December is one of my favorite times of year. Coming off of Thanksgiving and looking forward to the end-of-year holidays, it’s when I get
PHARMACEUTICAL QUALITY METRICS: Why You Should Be Concerned, And What You Should Be Doing Now
FDAnews Inspections Summit I recently had the opportunity to moderate a panel discussion on pharmaceutical quality metrics at the 10th Annual FDAnews Inspections Summit in
Our New Look: An Updated Brand Reflects Excellence, Innovation & Client-Centered Performance
It is an axiom of marketing that a company’s brand identity is fundamental to long-term business success. It is also well-known that, on occasion, a
FDA’s Inspectional Authority: Inviolate or On Shaky Ground?
I just posted a response on LinkedIn to a recent LinkedIn post by John R. Fleder (from his firm's excellent FDALawBlog) surrounding FDA's inspectional authority
Smile, You’re On FDA-Camera!
The following entry is an excerpt from a LinkedIn post that I responded to today. It pertains to FDA's authority to take photographs during inspections,
Welcome To The Party Compounders!
The September 12 edition of the industry newsletter QMN Weekly contained an article about objections voiced by the International Academy of Compounding Pharmacists (IACP) concerning
FDA’s Upcoming Quality Metrics Submission Requirement: Data Driven Decision-Making or Letting the Students Issue Their Own Report Cards?
Although Corporate America has embraced the concept of metrics and dashboards for close to 20 years now, the US Food and Drug Administration (FDA) has
The Coming Evolution of Quality Management Software — Maybe.
I had an interesting phone call today with a company named ZenDoc — www.getzendoc.com — a software company, headquartered in Dublin, Ireland. The company contacted me
Batch Failures: Significant Risk Events; Challenging Investigation Opportunities
Today's (4/25/2012) news from In-Pharmatechnologist.com (see full link below) highlights information from FDA's 2011 Warning Letter dataset. The article states that: "In fiscal 2011 the US
FDA Warning Letters Increase 155% from 2010 Levels
If you have any role within an FDA-regulated company, it is likely you are aware that the FDA has been much more focused on enforcement
New FDA Inspections’ Database — How Useful for FDA-Regulated Industry?
The new Inspections Database (http://www.fda.gov/ICECI/EnforcementActions/ucm222557.htm) is a positive step by FDA to provide further transparency for the public (and likely their Congressional representatives!) regarding enforcement
Urgency: Promise or Problem for FDA-Regulated Companies
A couple of months ago I was engaged in a brief, friendly dialogue with one of my close friends – a college buddy – about
UPDATE: Free FDA GMP / QSR Ebooks for IPhone, IPad
This is an UPDATE to a post from May 2010. Please click this link for the original post. On Monday, June 21, Apple released the
Webinar, June 23: Inspection Readiness for a More Aggressive FDA
Join the Center for Business Intelligence for a one-hour, information-packed webinar on managing FDA inspections from a more aggressive FDA. Now that pharmaceutical, medical device,
HACCP: Underused, Underappreciated and Exceptionally Powerful
“When everything is critical, nothing is.” Author Unknown When you think back on your career as a Quality & Compliance Executive, how many times have
Will Robinson – Soldier, Family Man, Professional, Friend – RIP
Memorial Day Week is a fitting time to learn of the passing of a professional colleague and someone that I am proud to have considered
Free FDA GMP / QSR Ebooks for IPhone, IPad, Blackberry & Other E-Reader Devices
Aren't the IPhone and the IPad great?? All of the information you could possibly want, right there at your fingertips! As Quality & Compliance professionals,
Two Billion, One Hundred Twenty Million Dollars – The Real Cost of Non-Compliance
I’m struggling to find an answer to the following question: If you were the owner of a company, how much would you spend, year over
The Risk of Using the GMPs to Design Your Quality System
Holmes on Homes Over the last few months, I’ve become a big fan of a TV show called Holmes on Homes. The show features super-hero
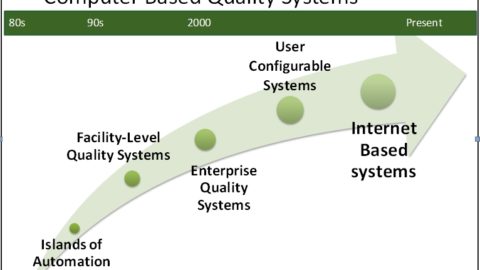
The Future is Now for Computer-Based Quality Systems
Click here to read Jack's article in GxP Lifeline by Master Control on "The Future is Now for Computer-Based Quality Systems" Text of Article Follows:
Admissions of Failure – A Lesson from Google for FDA-Regulated Companies?
Imagine seeing the following (imaginary) Wall Street Journal headlines: ACME Pharmaceutical Admits Product is No Better Than Competitor CRO Co. Report Shows Poor Clinical Program
Interview: FDA Inspection Readiness
Business Intelligence Solutions Interview with Jack Garvey FDA INSPECTION READINESS March 2010 What are the biggest issues currently facing FDA-regulated companies when it
Tick, Tick, Tick: FDA Steps Up Criminal Investigation Activity
A review of FDA enforcement news over the last year should clearly demonstrate an important shift in approach by the FDA that the “cop” side of
Compliance on a Budget: Can it be Done? What are the Risks?
Like a lot of people, when I was a younger, I always wanted the newest toy or the shiniest item in the store. An unfortunate
Outsourcing and Offshoring in a New Era of FDA Enforcement
On Thursday, March 11, 2010, Jack Garvey, Principal, Compliance Architects LLC, will be presenting an audio conference in conjunction with FXTranslations and FXConferences entitled "Outsourcing
Regulatory Alert: New FDA Proposed Rule — Sponsor Reporting of Falsified Data
Today the FDA has published a proposed rule in the Federal Register (75 FR 33 at 7412, 2/19/10) that will require sponsors (and defining petitioners
Implementing an FDA-Regulated Quality System Using Microsoft SharePoint®
New Jersey based pharmaceutical and medical device companies have a great opportunity this month to gain some significant information on how to improve their compliance
Bioanalytical Quality Standard Initiative
Jack Garvey, Founder of Compliance Architects LLC is Co-Chair of the Bioanalytical Quality Standards Initiative. This initiative, conceived by Synomics Pharma Services, was founded to
The Importance of Compliance Strategy
strat - e - gy [strat-i-jee] - noun skillful use of a stratagem: The salesperson's strategy was to seem always to agree with the customer.
Compliance Software Solutions & Compliance Outsourcing
Scale without cost. These are watchwords to modern business. I want my operations to scale with my business volumes, but I don’t want to add
FDA 483 & Warning Letter Response & Planning — DOs and DON’Ts
If you are reading this now, you are probably in the middle of an enforcement crisis. Responding to an FDA-483, Warning Letter, or in some
Pharmaceutical Company Subject of DOJ Sting, Story, Page 7
Byline 1/19/2010, Legal Times DOJ Undercover Sting Nets 22 in Large Scale Foreign Corrupt Practices Act Case If you are working in a senior legal,