We enjoyed our very own Teresa Gorecki, Practice Lead at Compliance Architects, speaking with Geoff Mix, Head of Content and Research for Executive Platforms, when we attended the Pharma Manufacturing World Summit 2023.
In this interview, Geoff talked to Teresa about the latest developments in FDA regulations post-pandemic in the United States. They explored the evolving landscape of cell and gene therapy and discussed critical industry insights. Read more to see what they discussed, or watch the video below.
Table of Contents
The FDA Regulations Post-Pandemic Approach
Geoff: What changes are we seeing from the FDA (Food and Drug Administration) as the pandemic winds down?
Teresa: The FDA’s approach has undoubtedly shifted. During the pandemic, inspections were halted, and they relied heavily on 4003 requests to gather documentation from companies. However, it appears they haven’t done much with the collected information.
Teresa: As they resume inspections, they’re returning to data from the last pre-pandemic inspections, sometimes dating back to 2018 or 2019. This results in more extended, more complex inspections with a higher volume of document requests and more investigators. There’s a noticeable increase in enforcement bias, possibly due to feedback suggesting laxity during the pandemic to expedite treatments.
Regulatory Changes and Industry Implications
Geoff: Are there specific regulatory changes the industry should be aware of?
Teresa: While we’ve seen some regulatory changes, they aren’t dramatic. The most significant changes allowed rapid vaccine and treatment approvals during the pandemic. The 4003 request process is new, allowing the FDA to request extensive company information. Companies need to be familiar with this process and parallel pathing for BLAs.
Impact on Different Sized Organizations
Geoff: How are these changes affecting different-sized organizations in the industry?
Teresa: The FDA’s willingness to expedite reviews for novel, life-saving drugs and rare disease treatments has pressured organizations of all sizes. Smaller companies often rely heavily on their CDMOs, facing challenges if they don’t choose the right partners. Large companies, while having the resources, struggle with their extensive processes and infrastructure. Medium-sized companies strike a balance, having enough infrastructure to be self-reliant but more nimble than larger entities.
Challenges in CMC for Startups
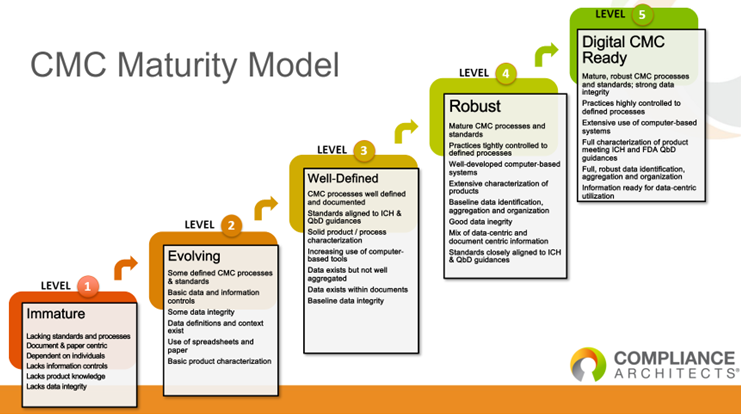
Geoff: Could you elaborate on the challenges startups face regarding CMC maturity?
Teresa: Startups have learned from the mistakes of predecessors. They often bring in a VP of CMC early to address how they’ll develop their technology and manage production. This early focus on CMC is crucial for navigating regulatory requirements and ensuring successful product development.
Supply Chain Risks in Cell and Gene Therapy
Geoff: Supply chain disruption is a significant concern. How does this impact the cell and gene therapy space?
Teresa: Supply chain risks are significant, especially for cell and gene therapies. The reliance on novel, research-grade reagents, often uncertified by suppliers, poses challenges. Startups must invest heavily in stability studies and characterization to meet FDA requirements, which are both costly and complex.
The Future of Cell and Gene Therapies
Geoff: What lessons can we learn from the early successes of cell and gene therapies?
Teresa: The biggest lesson is the cost and scalability of these therapies. With treatments often costing hundreds of thousands of dollars, there’s a push to reduce costs. Future advancements will focus on making these therapies more affordable and scalable. Technologies like exosomes and regenerative medicine are promising, but the key challenge remains to produce these therapies reliably and at lower costs.
Compliance Architects as a Partner
Geoff: How does Compliance Architects assist companies in navigating these challenges?
Teresa: We provide personalized, expert consultation tailored to each company’s unique challenges. Our senior-level subject matter experts, including myself, work closely with clients to understand their needs and develop innovative solutions. We specialize in complex regulatory environments, offering practical, experience-based insights to help companies succeed.
Key Takeaways
Geoff: What key points should our listeners take away from this conversation? Any final guidance?
Teresa: Remember that guidelines and standards often lag behind technological advancements. If you’re at the forefront, you must educate regulators and demonstrate compliance creatively. Also, leverage existing knowledge and research, especially from academic sources, to solve novel problems.
Getting in Touch with Compliance Architects
Geoff: How can interested companies get in touch with Compliance Architects?
Teresa: Our website, compliancearchitects.com, has contact information for all of us. You can also reach out via LinkedIn. We’re always ready to help address your regulatory and compliance challenges.
Geoff: Thank you, Teresa, for sharing your valuable insights. This conversation has been incredibly enlightening. For those listening, contact Compliance Architects for expert guidance on navigating the complex regulatory landscape.

Learn more about Teresa Gorecki here.
Contact us below for more insight into how we provide personalized, expert consultation tailored to each company’s unique challenges.





