In this 4 part series, we are talking through GxP Best practices
This article reviews the key highlights from Teresa Gorecki‘s Executive GxP training, Session 1: Differentiating Between Responsibilities and Accountabilities for Biopharmaceutical Companies with Outsourced Operations.
In this session, Teresa provides a deeper context for accountability and responsibility for biopharmaceutical companies with outsourced operations.
To learn more about Teresa, refer to the image below.

Table of Contents
Evolving Regulatory Landscape and Strategic Outsourced Operations
In pharmaceutical development, regulatory bodies like the FDA demand sponsors uphold product safety and efficacy from early development through commercialization. Compliance with Good Clinical Practices (GCP), Good Manufacturing Practices (GMP), ICH Standards, and FDA guidelines requires rigorous oversight of outsourced operations.
Over the past 25 to 30 years, regulations have been adjusted to meet industry changes. Outsourced operations have become pivotal, helping companies expand capacity and cost savings, improve efficiency, and access specialized skills. Virtual firms especially use outsourcing to focus on core strengths. However, lessons from inspections highlight the need for unified global standards and coordinating requirements across health authorities.
Navigating these regulatory shifts requires proactive oversight and adherence to evolving standards, ensuring product integrity and safety throughout its lifecycle.
Evolution of Sponsor Responsibilities in Clinical Trials
In the 1980s, under CFR 312, sponsors’ responsibilities in clinical trials included monitoring investigations, ensuring compliance with protocols and cGCP, maintaining an Investigational New Drug (IND) application, and documenting all responsibilities delegated to Contract Research Organizations (CROs) and other third-party providers.
Fast-forward to ICH E6 Revision 2 Good Clinical Practice (2016), sponsors’ responsibilities expanded significantly. They are now mandated to establish a comprehensive quality system distinguishing between Quality Assurance (QA) and Quality Control (QC) activities. The revision also emphasizes the critical need to monitor and oversee clinical trials, underscoring the sponsor’s pivotal role in ensuring trial integrity and compliance with regulatory standards.
This evolution reflects a broader industry shift towards more structured and accountable clinical trial management, which aims to safeguard patient safety and data integrity while adhering to rigorous regulatory requirements.
The Importance of Oversight in Clinical Trials
In clinical trials, oversight goes beyond monitoring. It includes actively supervising and ensuring rules are followed, and the trial process is honest.
To oversee means supervising responsibly, not solely delegating responsibilities and walking away. When outsourcing aspects of clinical trials, sponsors must commit resources to oversee delegated responsibilities effectively. This includes understanding the necessity of outsourced activities for patient safety, product quality, final approval, and the asset’s potential value if intended for divestment or profit.
Several critical factors must be considered:
- Disease State and Patient Population: Understanding the specific disease context and patient demographics impacted by outsourcing business functions.
- Quality and Compliance Profile: Evaluate third-party providers to ensure their quality systems, SOPs, and IT tools meet regulatory standards.
- Risk Management: Evaluating the execution of their quality systems and the associated risks and determining the acceptable level of risk tolerance.
- Resource Commitment: Considering the time, financial resources, and personnel needed to rectify any deficiencies in outsourced work that do not meet regulatory standards or company requirements.
Methods to determine the level of oversight needed include technical assessments, site visits, benchmarking, and conducting requests for proposals (RFPs). Regulatory compliance scans and quality audits also play crucial roles in ensuring adherence to standards.
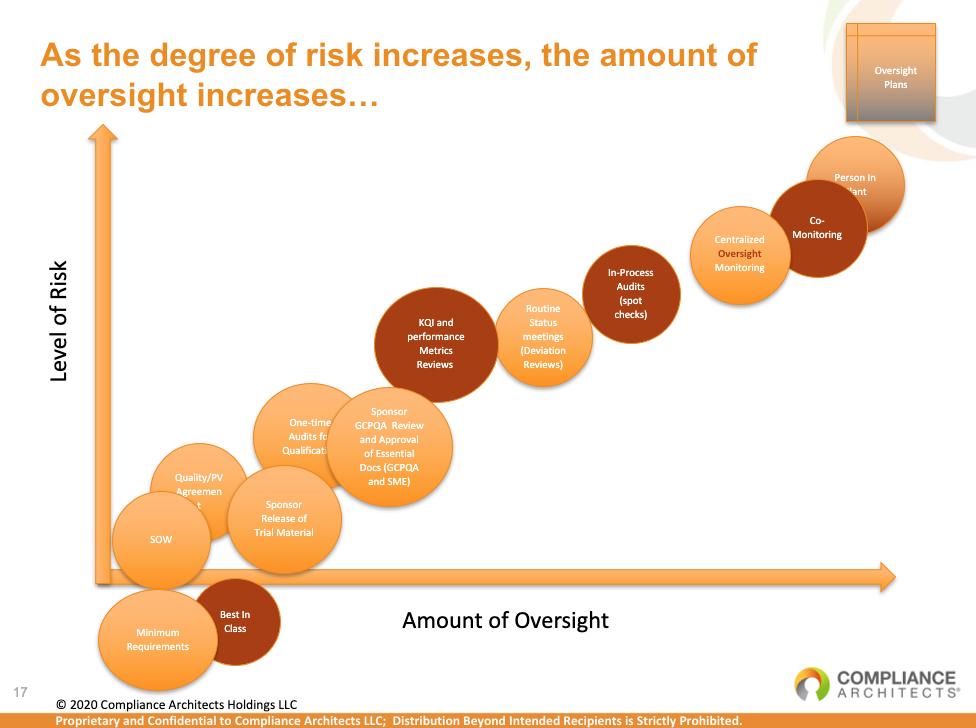
Effective oversight activities should include regular meetings to review key quality and compliance metrics, analysis of deviation reports, periodic spot-check audits, and co-monitoring visits. These measures are essential for maintaining control over outsourced activities and ensuring the successful execution and regulatory approval of clinical trials.
The Limitations of Audits for High-Risk Third Parties
While audits are critical for evaluating compliance and quality, they may not meet requirements for high-risk third parties like Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs). Regulatory frameworks such as CFR 312 and ICH E6 Revision 2 emphasize the sponsor’s responsibility to actively monitor and oversee all clinical investigations and product manufacturing aspects.
Audits provide snapshots of compliance at specific points in time. However, effective oversight demands continuous quality management throughout every trial or manufacturing process stage. This includes setting clear standards and expectations, reviewing and approving critical documents and reports, and ensuring the release of all products.
Moreover, high-risk third parties often operate under their own Standard Operating Procedures (SOPs) that revolve around their specific activities. Sponsors must establish and enforce standards that meet their requirements and regulatory guidelines. Selecting, approving, and ongoing oversight are essential to reduce risks and ensure adherence to high safety standards, efficacy, and regulatory compliance.
Therefore, while audits are essential oversight components, sponsors must provide robust systems for continuous quality management and proactive monitoring to effectively manage risks associated with high-risk third-party engagements.
Stay tuned for part 2 of this series to discuss GxP Session 2: Early Stage Tips for Data Integrity.
Please complete the form below to learn more about the accountability and responsibility of biopharmaceutical companies with outsourced operations.





