In the realm of quality and compliance within pharmaceuticals, biopharmaceuticals, and combination products, I’ve frequently been asked about the crucial steps to pass an FDA Pre-Approval Inspection (PAI). It brings to mind Steven Covey’s timeless advice from “The 7 Habits of Highly Effective People” – “Begin with the End in Mind.” This principle aligns perfectly with the preparation required for a successful PAI.
Having served as a Consultant and Practice Lead in this field, I’ve amassed a wealth of experience, allowing me to offer invaluable insights and strategies to navigate the intricate process of preparing for an FDA PAI.
Here, I’ll distill some expert tips from years of hands-on experience and industry knowledge to help you gear up effectively for this critical inspection.
Table of Contents
When should a company begin preparation for an FDA Pre-Approval Inspection?
The PAI begins as you move through the development phase when creating the documentation that will be part of the CMC Section of the NDA/BLA Submission to the FDA for a new product.
Why?
The CMC Section of the NDA/BLA Submission is prepared months, if not a year or more, before an FDA Pre-Approval Inspection. Critical sections of the development report submitted in the CMC section of the NDA/BLA, the documentation supporting the tech transfer and validation of the process, and QC testing methods are extensively reviewed during a pre-approval inspection.
The “FDA Compliance Program Manual for Pre-Approval Inspections” provides a framework for the FDA Pre-Approval Inspection Program, the overall process, and instructions on how the FDA executes the Program.
There are three primary objectives for an FDA Pre-Approval Inspection, each of which considers the potential risks identified during the review of the NDA/BLA Submission and the threat posed by the manufacturing facility(ies) based upon prior Inspection outcomes.
These objectives include:
- Adherence of the Production Process, Test Methods, etc., to the information provided in the CMC Section of the NDA/BLA Submission
- Facility Readiness for Commercial Manufacturing
- Data Integrity Audit
The FDA Investigator(s) conducting the Pre-Approval Inspection is tasked with assessing the adherence of the Production Process, Test Methods, etc., to the information provided in the CMC Section of the NDA/BLA Submission. This means the data generated to create the CMC Section of the Submission is subject to FDA review during the Pre-Approval Inspection.
The quality of the final stage of the development, technical transfer, and validation documentation needs to be created to withstand rigorous review by the FDA during the Pre-Approval inspection. This is why a firm must begin Process Development “with the end in mind.”.
FDA Expectations for Pre-Approval Inspections
In the “FDA Compliance Program Manual. for Pre-Approval Inspections,” the FDA summarizes their expectations regarding product and process design and how it impacts the risk to product quality, safety, and efficacy in the commercial manufacturing environment.
“Drug product design helps to determine whether the product can meet patients’ needs and maintain its intended performance through its proposed shelf life.”
In preparation for the Inspection, the FDA Investigators are instructed to review the CMC Section of the Submission before initiating the Inspection. Per the “FDA Compliance Program Manual. for Pre-Approval Inspections,” FDA Investigators are instructed to:
- “Become familiar with the chemistry, manufacturing, and controls (CMC) section of the application and related drug master files (DMFs) for the establishment to be inspected. If possible, review the pharmaceutical development section before initiating the inspection.”
- “Participate as appropriate in internal FDA Integrated Quality Assurance (IQA) Team meetings to provide or seek feedback on the application. Also, as necessary, discuss questions/concerns related to data reliability (e.g., test methods, 17 See MAPP 5014. Determine if other FDA IQA team members need data audit coverage of specific areas during the inspection.”
A key objective during the Pre-Approval Inspection is to verify that the formulation, manufacturing, processing methods, QC methods, and master production batch records are consistent with information in the CMC section of the Submission. This will likely include a review of tech transfer and process validation batch records and documentation, including the QC testing, microbiology/sterility testing, and stability testing completed to date.
In addition to these documents, segments of the Development Report may be reviewed during the Pre-Approval Inspection. In particular, the In-Process Controls, Bulk and Finished Product Specifications, and Stability segments will likely be referenced or reviewed by the FDA Investigator. Ultimately, the FDA Investigators conducting the Pre-Approval Inspection are tasked with assuring the accuracy and reliability of the information provided in the NDA/BLA Application.
Where do I see firms go wrong?
There are a few areas where I often see firms struggle.
Reports Not Stored Properly
The protocols and reports that capture the development of the process, test methods, and stability data generated during product development are often not stored in an easily retrievable manner. I often see these reports stored in file cabinets, on the hard drives of individual PCs throughout the firm, or unsecured “shared sites” on the firm’s network.
Protocol History Not Documented
The history of the changes to the formula, production process, in-process controls, specifications, and/or QC testing methods is not adequately documented.
Documents Not Well Organized
The documentation to support the development of seed stocks or viral vectors is not well organized and does not link to the clinical trial lots manufactured. This is also true for Reference Standards developed by the firm, which are critical to the biological and/or analytical test methods that quantify identity, purity, and potency/efficacy.
Production Site Changes Not Documented
The history of API and/or drug product production site changes is not adequately documented.
Change History Not Documented
The link between which site(s) clinical trial material was produced and the clinical trial outcomes is not adequately documented. This is not linked to the overall change history through the development process.
Development Report Doesn’t Tell a Story
The Development Report is poorly structured and is an unorganized “composite” of numerous studies required to support the CMC section of the NDA/BLA. It does not “tell the story” of the development of the product.
No Reports or Documents on the Tech Transfer Process
The protocols and reports supporting the tech transfer of the process and QC test methods are not often seen as a critical document package the FDA will review during the Pre-Approval Inspection.
All the documentation described above is part of the information that creates the foundation to support the product through its lifecycle. Regulatory Affairs creates the CMC section of the NDA/BLA, working from documents from many diverse sources.
In some extreme cases, there is no way to assure that the documents provided were indeed the correct documents because the documentation supporting the development of the product is in disparate places and is not well organized. That is a strong statement, but I have seen many situations where this IS the reality.
How does a firm know if they are well positioned to achieve a favorable Pre-Approval Inspection outcome for the first objective in the Inspection?
First, you should know that the first objective of the FDA is to adhere to the production process, test methods, etc., and the information provided in the CMC Section of the NDA/BLA Submission.
The firm should self-assess to understand how mature its Development Process is in supporting the development and submission of the CMC Section of the NDA/BLA.
Many organizations see the creation of the CMC Section of the NDA/BLA as a paper exercise driven by Regulatory Affairs. It is indeed much more than that.
I agree with the FDA’s statement:
“Drug product design helps to determine whether the product can meet patients’ needs and maintain its intended performance through its proposed shelf life.”
The critical activities and documentation of those activities during development, tech transfer, and validation at the commercial manufacturing site are essential to reliably supplying a finished drug/biological/combination product from launch through retirement that is safe and effective throughout its life in the marketplace.
CMC Maturity Model
So, where is your firm? Did you begin Development with “The End in Mind?” The end being a successful Pre-Approval Inspection as one of the criteria for a successful end?
I have created a CMC Maturity Model based on what I have seen throughout my career in the Industry and as a Consultant. It is presented below.
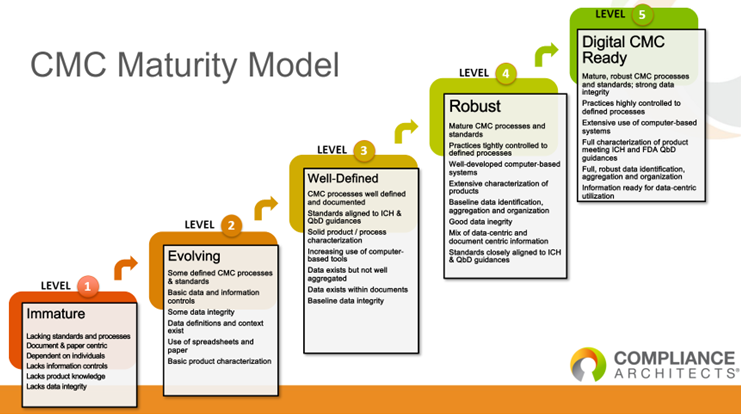
At Level 1, the firm will struggle to adequately support the documentation requests and discussions with an FDA Investigator(s) during a Pre-Approval Inspection. The outcome of this Pre-Approval Inspection could potentially result in numerous 483 observations or even a withhold approval inspection rating by the FDA.
The firms that can successfully navigate the Pre-Approval Inspection smoothly are those at Level 3. Barring any significant GMP issues, a firm at Level 3 will be able to conclude the Pre-Approval Inspection with approval of the site for commercial manufacturing of the new product.
Those firms at Levels 4 and 5 are not only able to efficiently support the intensive document review from development through tech transfer and validation at the commercial manufacturing site, but they will also able to support the changes needed post-commercialization, which will support future FDA Inspections post approval of the NDA/BLA. After the Pre-Approval Inspection, they will have no difficulty receiving an approval rating for the commercial site from the FDA.
With that in mind… I will go back to my initial question…
When should a company begin preparation for an FDA Pre-Approval Inspection?
During the development of the product, process, and test methods.
The Development Organization must understand that each and every experiment, test, protocol, and/or report may potentially be inspected by the FDA months to years after the work was done.
Organization of that data and documentation is essential; ensuring the correct information is submitted to the CMC Section of the NDA/BLA is critical. The link between the clinical trial product, what will be launched commercially, and the history of changes throughout clinical and product development is essential. The Development Report should provide a well-organized summary of the history of the development and evolution/changes to the formula, process, test methods, primary container/closure, site(s) of manufacturing, primary production equipment, etc., for the product.
What should the Commercial Site Team have available for the Pre-Approval Inspection to fulfill FDA’s first objective for the Pre-Approval Inspection?
Adherence of the Production Process, Test Methods, etc., to the information provided in the CMC Section of the NDA/BLA Submission.
The site team should have the information to support the final commercial manufacturing package, including the master batch record and in-process controls, QA test methods, specifications, and product expiration dates. This includes tech transfer and validation of the production process and QA test methods at the site. It also includes the stability data submitted in the NDA/BLA and the proposed marketed product stability protocol.
How should the firm prepare for the first segment of the Pre-Approval Inspection, which will assess the firm’s adherence to the production process, test methods, etc., to the information provided in the CMC Section of the NDA/BLA Submission?
I would recommend the following to prepare for this segment of the Pre-Approval Inspection.
- Determine your CMC maturity level. The level that your firm is at will inform how early your firm needs to begin to prepare for the upcoming Pre-Approval Inspection. The higher the maturity level, the less time required to prepare.
- Assemble a “PAI Readiness Team” comprised of essential subject matter experts from development, analytical development, tech transfer/tech operations, manufacturing, plant QA, QC, and QA compliance. If the product is produced by a third party or a third party conducts QC/Micro testing, create a cross-site team to ensure adequate support at the third-party location for the Pre-Approval Inspection; present a united front to FDA.
- Review the CMC Section of the NDA/BLA as a team, along with the Development Report and key Reports that the FDA will likely request during the Inspection.
- Invite an independent third party (from within or outside the company) to conduct a “mock PAI” with the PAI Readiness Team. Optimally, this should be done twice. Once early in the readiness process, any critical gaps found during the mock PAI can be addressed before the FDA Pre-Approval Inspection.
- The second mock PAI should be a dress rehearsal with the subject matter experts who will present to the FDA. This will provide an opportunity to coach the subject matter experts on managing their interactions with the FDA during the Pre-Approval Inspection.
- Invite the company Compliance organization to conduct a data integrity audit of the data and documentation that the FDA will likely request during the Inspection.
- Organize all the documentation before the Inspection so it is readily available in electronic and hard copy format.
- Develop an overview in PowerPoint of the development history, including the history of changes to the process, test methods, specifications, etc., to be presented to FDA during the opening of the Pre-Approval Inspection.
What is the most crucial thing your firm can do to be “really ready” for an FDA Pre-Approval Inspection?
“Begin with the End in Mind”…
- Understand how mature the CMC process is within your firm. Request a third-party assessment from one of the Q&C Consulting Firms with Consultants who understand the Development/CMC process. Compliance Architects may be able to assist you with this assessment.
- Utilize one of the newest IT solutions available to organize all of the developed documentation needed to support the CMC Section of the NDA/BLA, understanding that this critical information is the foundation upon which the quality of the product will be managed through its life cycle. One product that I recommend is QbDVision from Cherry Circle Software.
- Develop a culture within the Development Organization within your firm that deeply understands the importance of the documentation created during the development process and how it forms the foundation upon which the product will be managed throughout its lifecycle.
Ready to take that first step in understanding where your organization fits on the CMC Maturity model? Contact us below!





