
The FDA-regulated industry has faced a persistent issue for years: writing skills are not prioritized. Quality and operations personnel are rarely trained to write!
This is especially concerning since FDA investigators assess a company’s compliance based on its written documents. Despite this, most companies do not provide writing training. While many attempt to navigate inspections through verbal explanations, you can’t talk your way out of poorly written documents.
As every industry professional knows, the FDA maintains that “if it wasn’t documented, it wasn’t done.” However, what’s often overlooked is that “If the FDA investigator can’t understand what you did, why you did it, and why it aligns with FDA requirements and expectations, it might as well not have been done at all.”
After surveying course participants for over ten years, data shows that the majority of people tasked with writing and reviewing compliance-related documents have received little to no specific training in writing skills. Among previous respondents, 66% had one or fewer college-level writing courses, and 85% had one or fewer business writing courses. Yet, two-thirds of respondents recognize compliance writing as a significant challenge, and only 10% of writers consider themselves experts—despite over 40% writing frequently and 58% reviewing documents regularly.
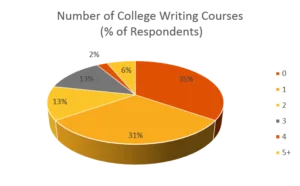
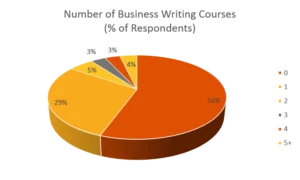
We don’t think it’s a stretch to ask: Could this be why we often have significant problems during FDA inspections?
© 2009-2025 Compliance Architects Holdings LLC – used by permission. All copyrights, trademarks and other intellectual property are the property of Compliance Architects Holdings LLC and are used by permission.