If you have any role within an FDA-regulated company, it is likely you are aware that the FDA has been much more focused on FDA warning letters enforcement activities since 2008. Alternatively, if you are involved at all with supply chain, manufacturing, quality assurance, quality control, regulatory affairs or other FDA compliance activities, you are likely intimately familiar with the FDA’s aggressive posture with respect to regulatory compliance enforcement. For people that spend their days working inside companies trying to build compliance systems and remediate compliance deficiencies, it common knowledge that FDA is looking harder, looking deeper, and issuing more FDA warning letters more than ever before.
However, even though I spend my days doing this sort of work I was shocked at a number that was just communicated to me in a public setting relative to FDA enforcement.

NYSBA’s Annual Meeting
On Thursday, January 26, 2012, I attended the New York State Bar Association’s Annual Meeting in New York City, and as part of that, attended the Food, Drug and Cosmetic Law Section meeting. As part of the regular Food and Drug Administration Update portion of the meeting, the Section was privileged to secure Elizabeth H. Dickinson, Esq., Acting Chief Counsel of the Food and Drug Administration, as the keynote speaker. As part of Ms. Dickinson’s talk, she briefly reviewed the FDA’s recent enforcement history.
This was where the shock came.
Ms. Dickinson outlined the number of FDA Warning Letters issued in 2009, 2010, and 2011. The numbers related by Ms. Dickinson during her talk are provided in the table below (I have added the % increase column).
FDA Warning Letters Data
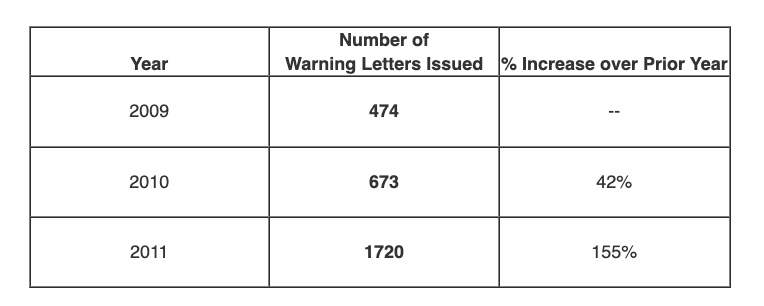
As can be seen from the numbers, FDA issued 1,720 FDA Warning Letters in 2011 – a full 155% increase over 2010 levels. This is also a full 262% increase over 2009 levels – an extraordinary increase by any measure.
Now, I must caveat that I have no idea whether these numbers are the final numbers that will be issued formally by the FDA for 2011 FDA Warning Letter levels. I further have not had the ability to directly verify these numbers with anyone else within the FDA. However, I think a statement by the Chief Counsel in a Bar Association meeting is a public-enough forum to feel comfortable sharing this information with a broader audience.
The continuing lesson for FDA-regulated companies is that the FDA is fully-engaged, and highly-focused, on enforcement activities. For those companies that don’t want to spend any more money on improving their quality and compliance systems because of “business” considerations, keep the following in mind: an FDA inspection is in your future – it is a matter of when, not if, FDA will walk through your doors. The costs of remediating compliance after the fact (and salvaging your company’s reputation, product brand equity, and loss in shareholder value) far exceeds the incremental spend on additional personnel, systems remediation and consultants in an ongoing manner.
Contact Us
So be careful out there – and for expert assistance with achieving best-in-class quality, compliance systems and responding to FDA warning letters, contact Compliance Architects by filling out the form below.





