In today’s rapidly evolving regulatory landscape, ensuring a robust quality culture is essential for any organization in the biopharmaceutical industry. Our latest infographic, “NAVIGATING YOUR QUALITY JOURNEY,” offers a clear visual guide on how Compliance Architects can help you map out your quality strategy over the next three years. See the infographic below.
Table of Contents
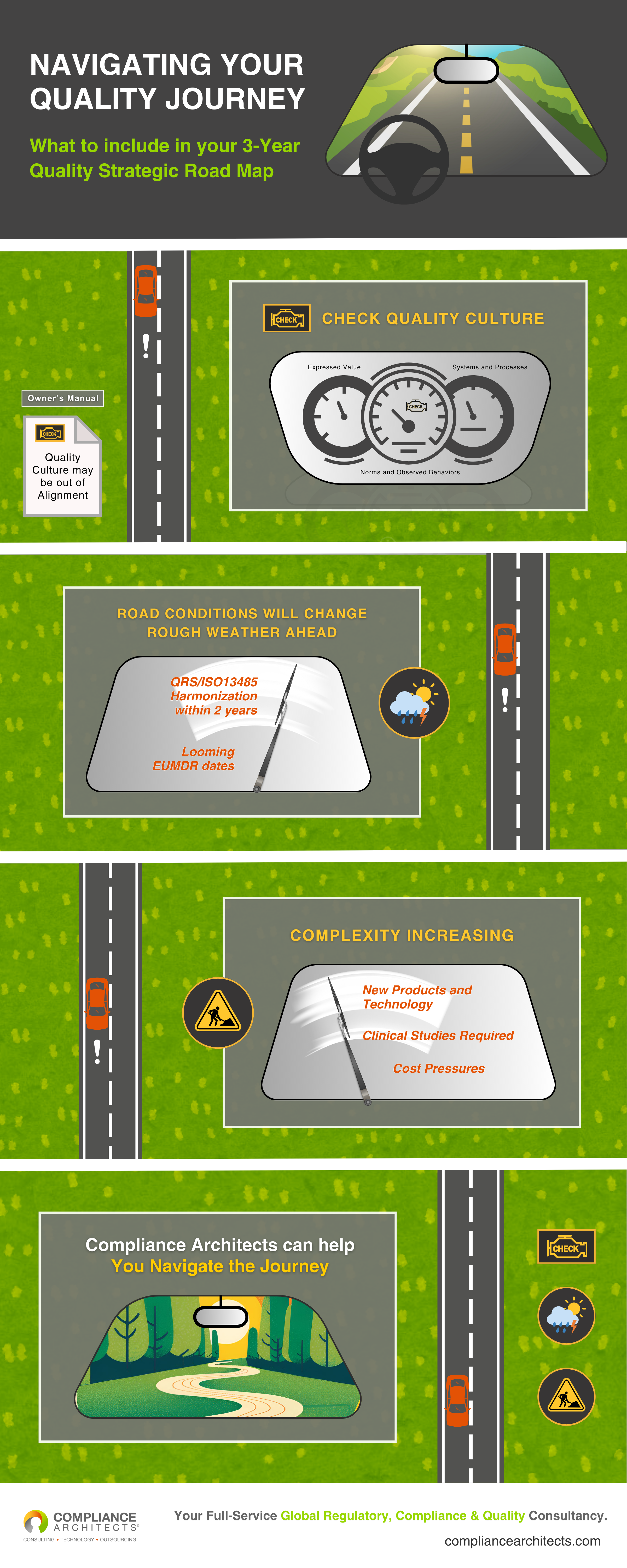
The Journey Begins: Check Your Quality Culture
Imagine driving down a clear road when suddenly, an alarming warning light flickers on your dashboard: “Check Quality Culture.” This alert is your cue to evaluate your organization’s expressed values, norms, observed behaviors, and existing systems and processes, as shown on the dashboard. A strong quality culture is foundational for compliance, and it’s essential to ensure that all aspects of your organization align with these values.
The owner’s manual metaphorically suggests that your quality culture might be “out of alignment.” This is a pivotal moment to assess and realign your internal practices, which sets the stage for the upcoming challenges ahead.
Navigating Rough Weather: Preparing for Change
As you continue on your journey down the road, the dashboard warns of “rough weather ahead.” This serves as a reminder that you must comply with critical regulations, including QRS/ISO 13485 Harmonization and looming European Medical Device Regulation (EUMDR) deadlines within the next two years. In order to clear your windshield, you must address these factors to clear the levels of compliance.
Ensuring compliance with these standards is not just a regulatory necessity; it’s a proactive step to fortify your organization against potential disruptions. Our expertise at Compliance Architects can help guide you through these complexities, ensuring you’re prepared for any regulatory storms.
Overcoming Increasing Complexity
Driving further along the road, another warning pops up: “Complexity Increasing.” This signals the need to adapt to new products and technologies, navigate cost pressures, and manage clinical studies. Each of these elements adds layers of complexity that can continue to clear your path to compliance.
To fully clear your view and successfully navigate these challenges, it’s crucial to implement a comprehensive strategy that addresses each layer of compliance. This includes not only understanding regulatory requirements but also integrating them into your organizational culture and operational processes.
The Reward: A Clear Road Ahead
Once you’ve addressed each compliance layer, your path clears, revealing a beautiful winding road bathed in the warm glow of a sunrise. Above your windshield, the message reads: “Compliance Architects can help you navigate the journey.”
Our team is dedicated to partnering with you every step of the way, ensuring that your quality strategic roadmap is not only clear but also sustainable. With our support, you can transform compliance from a daunting obstacle into a strategic advantage, allowing your organization to thrive in a competitive landscape.
Navigating Your Quality Journey with Compliance Architects
Navigating your quality journey is essential for long-term success. By recognizing the importance of a strong quality culture, preparing for regulatory changes, and addressing increasing complexity, you can ensure a smoother ride ahead. With Compliance Architects by your side, you can confidently face the road to compliance, knowing that together, we can illuminate your path to success.
For more insights on creating your 3-Year Quality Strategic Road Map, explore our infographic and reach out to our team of experts for personalized guidance. Let’s embark on this journey together!
Contact Us
To learn more about navigating your quality journey with Compliance Architects, contact us by filling out the form below.





