We believe that culture poses a direct risk to life science companies. By understanding your culture and having a predictive diagnostic model that you can use to assess your needs, see where improvements are needed, and target your remediation to that culture, you can inherently reduce your risk and tangibly lessen the risk for those organizations.
This article will detail two different quality culture scenarios, both good and bad. If you wish to watch the video instead, feel free to watch the video below.
Table of Contents
Healthy Quality Culture
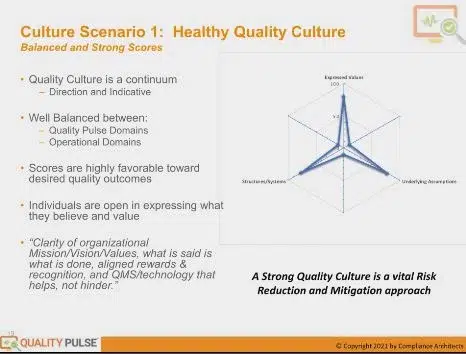
So, let’s look first at a healthy quality culture. If your company was doing this assessment and you described it as a healthy culture, this is what you would want to have. Having these diagrams orients you. This is a radar plot.
At the top is the expressed value score. Underlying assumptions are on the right-hand lower, and systems and structures are on the left-hand lower. And the higher the score, the longer these arms extend, and the healthier your culture will be.
It’s a quick way to get a look at your culture in aggregate across the organization. We’re not going to talk about sub-populations at this level, but this is a healthy score. Here are the reasons why we view this as a healthy score.
These scores are relatively high. They’re represented on a 1-10 scale, so these scores are 8s, 9s, and 10s. What’s particularly important is that the scores are balanced between all three and are strong.
It means that you have favorable outcomes on all the scored items. It’s well distributed across the organization. This indicates an organization with a high degree of clarity in the organizational mission, vision, and values.
What’s said is what is done. You reward people for doing those things. Your rewards and recognition align with what you want to accomplish concerning quality and your ways of operating.
This helps you achieve those goals instead of hindering them. You have a great deal of clarity in that. This type of culture significantly reduces and mitigates risk because everyone is pulling on the same rope in your robust quality systems. There’s alignment in decision-making.
Weak Quality Culture
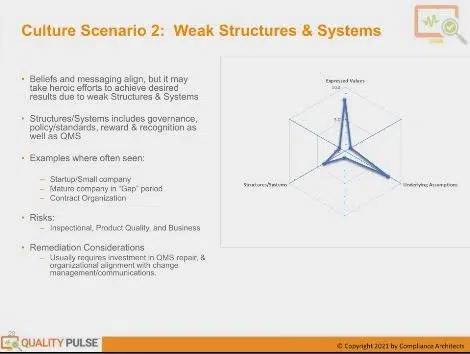
Let’s look at an example where you have one of these: weak. And this is probably one you’re most familiar with because it’s the most tangible. Also, if you exclude expressed value and underlying assumptions from your daily assessment, it would appear as an audit because an audit will only look at this systems and structure piece.
They won’t look at your cultural pieces from these other two culture vectors. So this is one where you may have a company with highly expressed values, and the belief systems may align very well with it. Everyone wants to do those things, and you have leadership support. But you have procedures and policies and structures and systems, including your rewards and recognition systems, that don’t help you do that.
It takes heroic efforts, often by a select few incumbents with a great deal of experience operating in this and the knowledge base, to achieve everyday results. Because you have these weak structures and systems, you rely on that individual effort rather than the structures and systems.
This is something you see very often in startups. You’ve got a tiny group of people who are very tightly aligned. Many of the decisions are made around the cafeteria lunch table. That intuitive exchange occurs unconsciously over time and becomes your system rather than your procedure.
It can also happen in a mature company during a gap period. You may not spend the time updating your procedures because you’re focusing on cost-cutting measures. If you have products going off patent and don’t have something to fill that gap period, you may be distracted by it.
Or it may be a contract organization where the margins are so small and tight that you must be laser-focused on that margin basis and your budget. Sometimes, this distracts from doing the right thing in difficult times. You can’t make those investments to improve your systems. You’re too busy getting stuff out the door.
Are you interested in learning if your organization has a good or bad quality culture? Take a look at our Quality Pulse product to see where your organization stands overall in terms of quality culture.
Unveiling the Quality Pulse®: Revolutionizing Quality Culture in FDA-Regulated and Life Sciences Companies
Understanding and cultivating a robust quality culture is paramount in the dynamic world of FDA-regulated and life sciences industries. Enter the Quality Pulse® quality culture diagnostic, a cutting-edge, research-based assessment model tailored specifically for these sectors.
The Foundation of Quality Pulse®
Rooted in rigorous scientific research, the Quality Pulse® model draws from a wealth of well-established principles and studies:
- Organizational Culture Principles: Inspired by Edgar H. Schein’s work at MIT/Sloan, this model delves deep into the core values and beliefs that shape corporate behavior.
- System Dynamics Principles: This approach leverages insights from Peter Senge of MIT/Sloan to address the complexities and interdependencies within organizational systems.
- Extensive Research: The model is built on dozens of research articles focused on corporate culture, quality culture, and organizational behavior, particularly within the context of life sciences, from 2000 to the present.
- Decades of Experience: With years of direct experience in quality operations across a diverse range of life sciences organizations (pharma, device, biotech) and company sizes (100-100,000 employees), the model is both comprehensive and adaptable.
Why Quality Pulse®?
The Quality Pulse® diagnostic is designed to help organizations, regardless of size, gain deep insights into the drivers of their quality culture. By doing so, it empowers company leadership with actionable intelligence, enabling them to:
- Direct Improvement Programs: Understand the specific areas that need attention and implement targeted strategies.
- Enhance Quality Outcomes: Improve quality results by fostering a culture prioritizing excellence.
- Elevate Organizational Excellence: Drive organizational performance by embedding a strong quality ethos.
In essence, the Quality Pulse® is more than just an assessment tool—it’s a catalyst for transformative change, guiding FDA-regulated and life sciences companies towards a future of unparalleled quality and success.
Learn more about Quality Pulse Here.
Transcript taken from June 15th Webinar – “Create a Quality Culture to Aid Risk Management: Dynamic Behaviors that Achieve Quality and Business Objectives”
Please fill out the form below to learn more.





