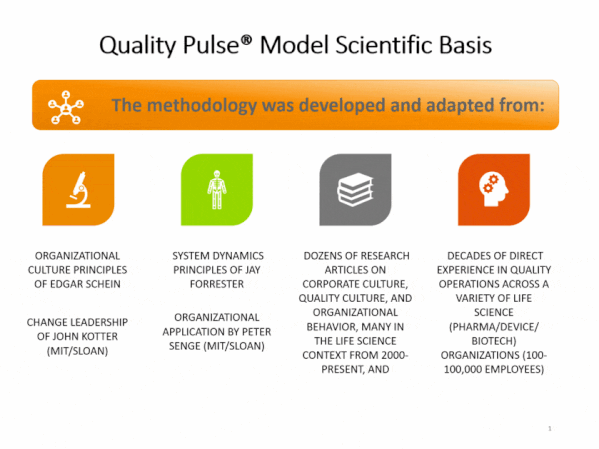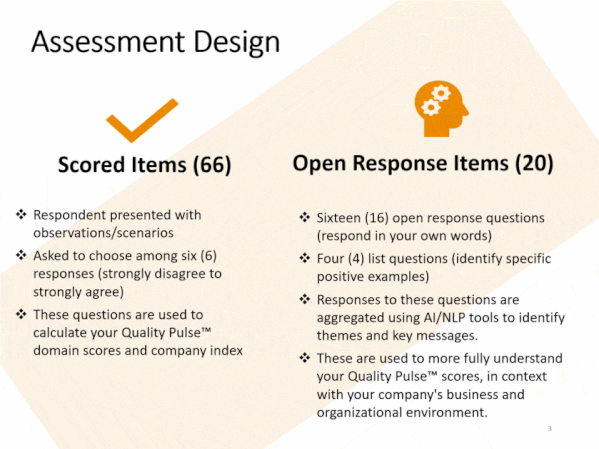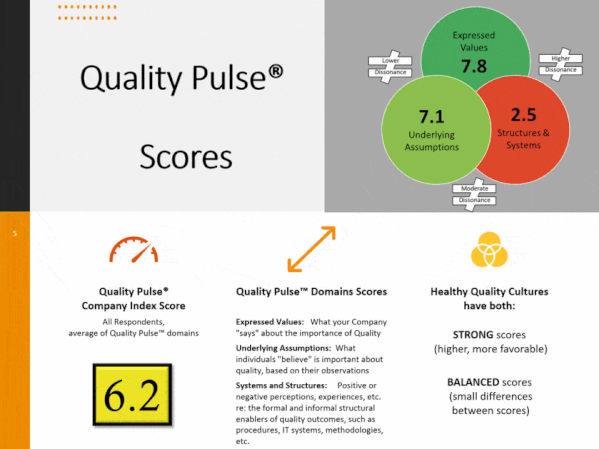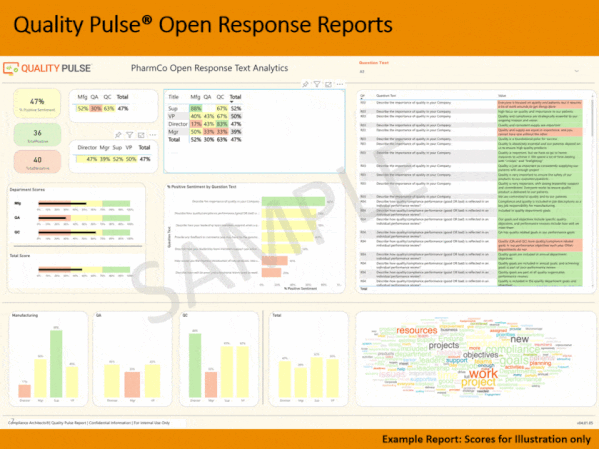
Quality culture is one of FDA-regulated industry’s most discussed topics. Understanding the drivers of behavior behind quality and compliance outcomes can be frustrating and challenging. Stated Corporate intent and policy can be disconnected from actual employee conduct. Pockets of information can lead to lack of transparency, and to personnel not working together as a team towards common goals, and with common understandings.
Compliance Architects® has developed industry’s first plant-floor and staff-focused innovative methodology, based on proven cultural survey science, to assess staff and line employee perceptions of quality culture – and its potential impact – by assessing and measuring individual and aggregate employee responses to fact-based questions scenarios simulating actual business situations.