The following article covers a transcript taken from June 15th Webinar – “Create a Quality Culture to Aid Risk Management: Dynamic Behaviors that Achieve Quality and Business Objectives”. The transcript covers a new quality culture paradigm section from the webinar.
These two slides date back almost 20 years, and the FDA intuitively saw that culture has a tremendous impact on the outcomes of life science companies, just as Teresa described.
They intuitively and empirically described what they saw as driving what creates culture. Those daily decisions are somehow linked to building a strong culture or creating a weak culture. And some of the attributes of that quality culture when it’s healthy, as well as the direct risks associated with it.
What we wanted to do, in looking at how to engage a culture paradigm, is how can we take those intuitive observations, or empirical observations of, here’s good culture when we see it, there’s not such a good culture, and create a quality culture model? Or a new way of looking at culture that we can then apply in the field to actual clients in actual operations that can assess culture, diagnose the issue, and then predictably and reliably take action that will improve culture.
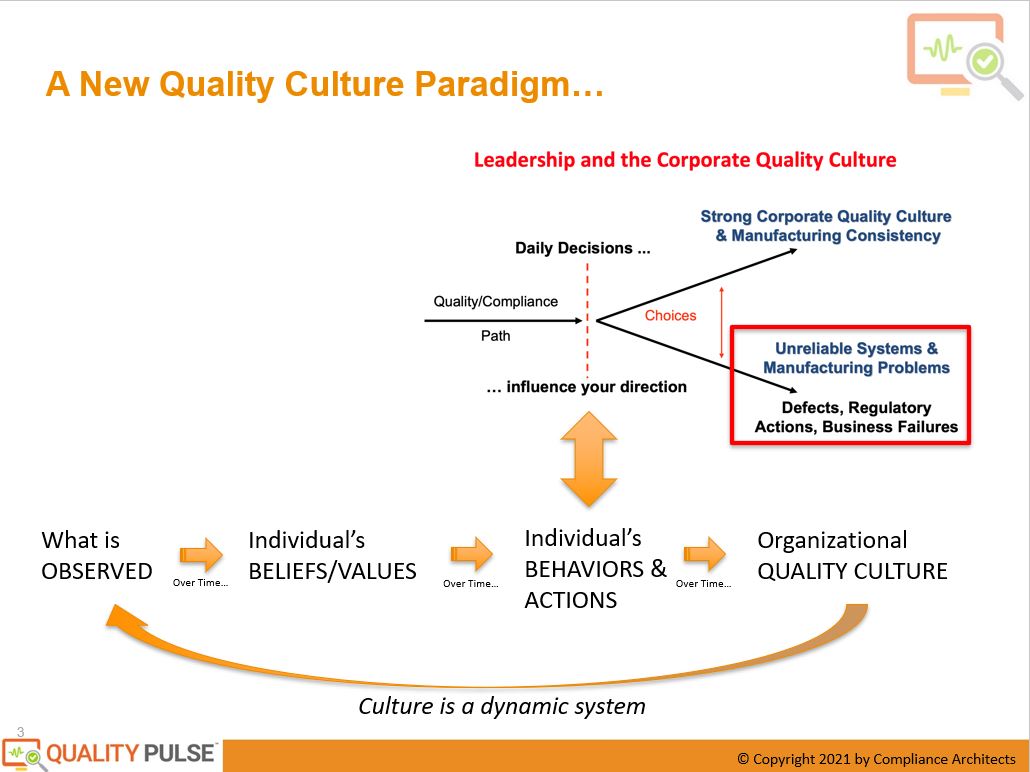
That took a little work and a lot of digging to understand where these daily decisions originated. Suppose you’re a worker at a life science company, particularly in the quality, manufacturing, or regulated areas, particularly in GMP or the filings area. In that case, individual daily decisions are driven by your personal beliefs, behaviors, and actions. That’s what is going to inform your decision-making. What have I been trained in? What do I believe is true? What will affect my job performance, goals and objectives, and the company’s stated goals and objectives? Those are driven by critical things developed over time: the individual’s beliefs and values within that operating context. If you’re operating in a company or an organization, that organization will influence your beliefs and values over time.
What is driving that belief and value creation? It’s what the individual observes in that environment. So if I’m operating, and this is true in our careers, if you’re working in an organization. You keep things around you, how your manager behaves, how your peers act, and how your leadership behaves under ambiguity and stress. Are they making a decision or working in a way that is aligned with your stated, observed objectives and values? Or are they doing something else? Or how are they reconciling that? What an individual keeps in that will inform their beliefs and values. As Rick Friedman described, those beliefs and values will then drive how that individual makes their daily decisions and which path you take, whether solid or weak corporate culture. It will be caused by that and as the result of the organizational culture.
Perhaps the most critical piece here is this is not a linear diagram. This is, in fact, a dynamic system. In researching this, among the literature, this is perhaps the most significant leverage point in understanding quality culture. What individuals observe over time creates their belief systems, what’s important, what they will be rewarded for, and what they should do. That will drive their behaviors and actions, and over time, that reinforces the quality culture because other people will observe what they’ve done. This is the new paradigm that was the basis for developing Quality Pulse.
Quality Pulse

The Quality Pulse® quality culture diagnostic is a scientific, research-based quality culture assessment model designed specifically for FDA-regulated and life sciences companies. The methodology was developed and adapted from well-established research, including:
- Organizational culture principles of Edgar H. Schein (MIT/Sloan);
- System dynamics principles of Peter Senge (MIT/Sloan);
- Dozens of research articles on corporate culture, quality culture, and organizational behavior, many in the life science context from 2000-present; and
- Decades of direct experience in quality operations across a variety of life science (pharma/device/biotech) organizations (100-100,000 employees).
Developed specifically to help organizations of all sizes understand the drivers of their culture of quality, the methodology and outcome reporting provides actionable intelligence that helps company leadership direct and implement improvement programs that will result in better quality outcomes and improved organizational excellence.
To learn more about Quality Pulse click here.
Contact Us
To learn more about quality culture or Quality Pulse, fill out the contact form below to get in touch with our team!





