Edgar Schein was a researcher in the 1950s and ‘60s in organizational dynamics and came up with a model that included what the organization says and what the organization does as principle drivers for creating a corporate culture. He put on top of that, if you’re familiar with change management you may recognize the name of John Kotter, who does most of his work out of MIT/Sloan. He’s written a lot of leadership books, particularly around leading change, which is his marquee book that he’s written. And it’s recommended reading for a lot of reasons, not just on culture, but on how to lead through change. A colleague of his at MIT/Sloan was Peter Senge in the ‘90s and in the 2000s. He did a lot of work on organizational dynamics and system dynamics. That causal-loop factor that you saw in that is a representation from Peter Senge, who derived his principles from Jay Forrester, post-World War II in some of the think-tanks that came out of the military department.
We put all of that research together along with the expertise that Teresa brought to the table, myself and some other colleagues at Compliance Architects. What’s unique about quality culture is that that organization operates within a regulatory framework that puts certain constraints on behaviors. We have to operate with a change control system, and with a deviation management system and a CAPA system. And our access to market is driven by approval of a regulatory filing, an application. That has to be driven by a research study. All of those business constraints that have to be done and overseen by a regulatory context put a constraint around that organizational structure that has to be operated and accounted for in that. So what Quality Pulse does is take these pieces, combine them very concisely, into something that then becomes this model. We’ll walk briefly through this because this is going to inform a lot of the conversations we’re going to have throughout the afternoon today.
Quality Pulse is predicated on the first premise, that observed behaviors of a group, which is another name for the culture, are the direct result of the belief systems of the individual members, represented right here in the middle of this diagram. Those belief systems are part of a dynamic system within the organization and are created, they are reinforced, or they are eroded and diminished by what individuals see and observe and experience within the organization. And there are three basic domains, or what we call a vector, by which you can influence this quality culture within a life science company. Let’s look at those three.
First off you have expressed values; what the company says. This is the posters on the walls. These are the quality standards and quality policies you may have. The key attribute to identify something that’s informing the expressed values of a company, you can usually see it. It’s tangible. It’s easy to identify. It’s very explicit. And it’s demonstrative. Those are pretty straightforward.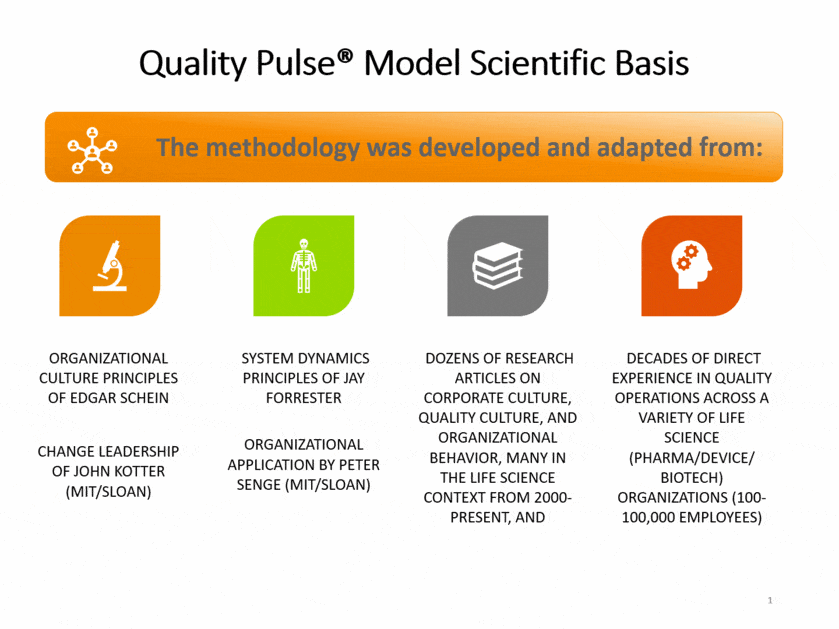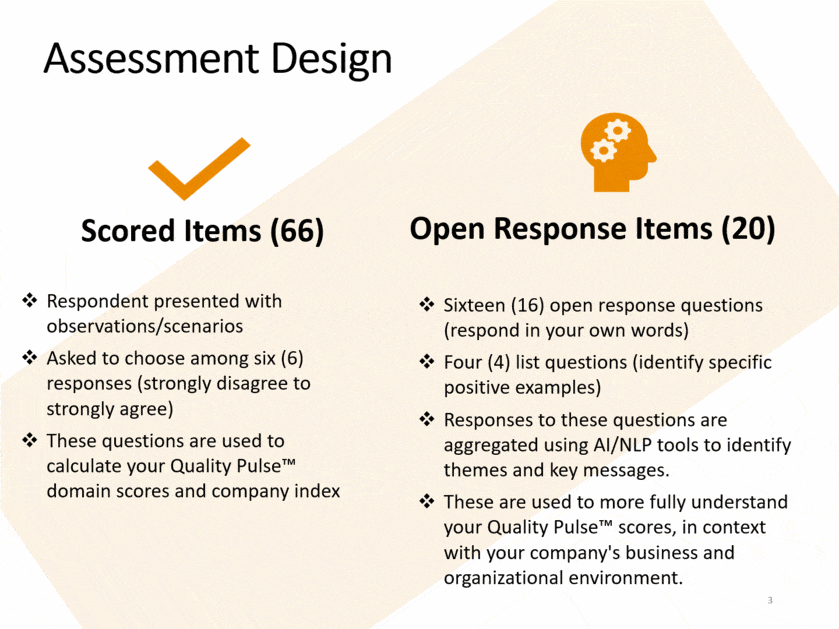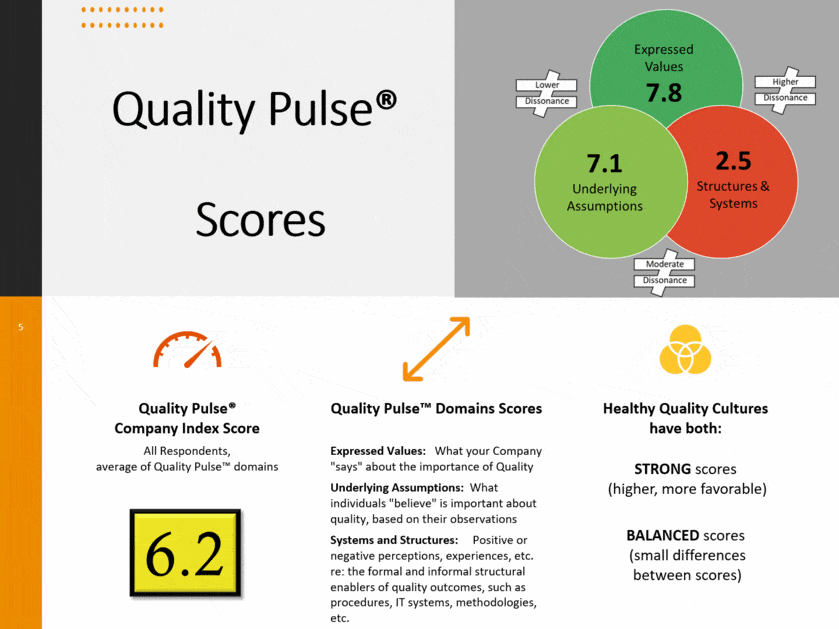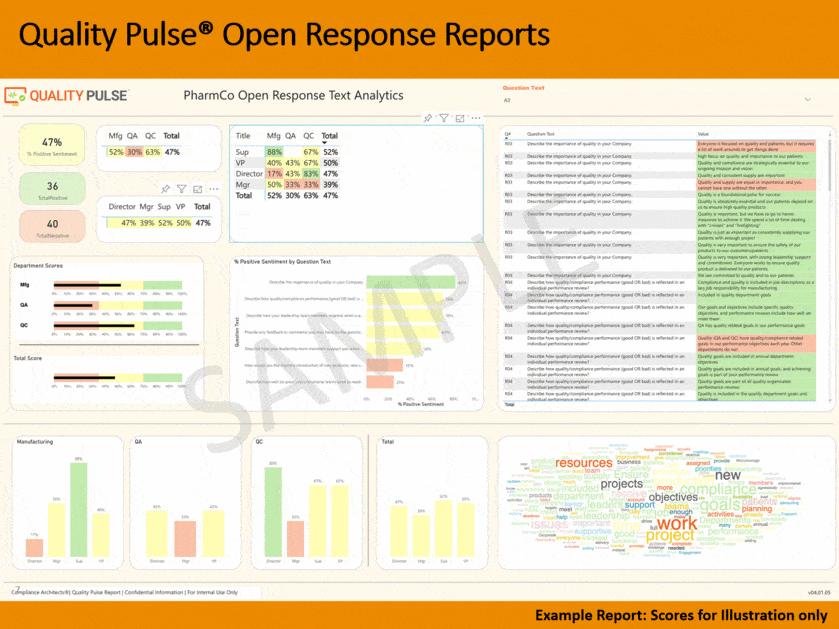
Underlying assumptions is where a lot of the leverage in culture is either created positively or created negatively. Underlying assumptions are what individuals believe is important about quality, based upon their own experience and observation within that organization or environment. You heard the term walking the talk. And people observe, how well do their leadership, how well do their peers, how well do their direct supervision, how well do they walk the talk? Do their individual actions align with what they say? That is going to drive individuals’ underlying assumptions. And it is particularly in frequency, consistently and authenticity when those actions occur. If it happens very often, and it’s very authentic, and you get consistent output, you’re going to have a tremendous synergistic effect on creating an underlying assumption or the belief systems that individuals see. What’s also particularly important in life sciences is how well you can do that during a difficult or ambiguous circumstance. If a company is under pressure, some of the examples that Teresa explained earlier. A company has external pressures. They need to supply more. We’ve all seen examples of it in our daily lives in the last year of, a company needs to produce more in a shorter period of time. How do you do that without compromising your principles? And when you have a circumstance or a scenario or something happens where getting it out on time and getting it out with quality come into conflict, the leadership behaviors and the demonstrated activities that individuals demonstrate, in those moments are going to be highly influential in creating culture. This is widely acknowledged as one of the most important pieces, particularly by Schein. But it’s also one of the most difficult to assess directly. So we have to go at this in some indirect ways to get this. And we leverage some technology with Quality Pulse which we’ll talk about later on.
Third is one that most of us are probably most familiar with, is systems and structures, because it’s tangible and it usually aligns for the most part with quality management systems. These are the ways of operating our processes and our procedures and our technology, very often the same ones we use to carry out our GMP functions. And how well do these structures and systems help you do your job consistently, align with your quality objectives, and take out that degree of difficulty in doing your work and doing it well and doing it right? Do you have antiquated systems that are aligned with a different quality paradigm, or different quality outcome? You’re going to see some friction in the execution of daily tasks. That erodes quality culture because your system is not aligning with your stated objectives.
The key point is that these three vectors do not exist in siloes. A single activity can impact multiple vectors. And these are all interdependent.
Transcript taken from June 15th Webinar – “Create a Quality Culture to Aid Risk Management: Dynamic Behaviors that Achieve Quality and Business Objectives”

