Navigate FDA Warning Letters: 4 Main Causes from John Daley
This White Paper provides an in-depth review of contemporary capabilities and solutions for effectively integrating third-party CMOs and other suppliers with GMP manufacturing quality systems and operations. It explores problems with quality oversight in the third party supply chain; how to think differently about managing the third-party supply chain; considering a technology framework to support improved third-party supply chain effectiveness; and how to “make it happen” for your company. This White Paper will stimulate your thinking and engage readers with questions about changing the game with people-process-technology third party supply chain approaches.
Managing the New Approach: Quality System Documentation
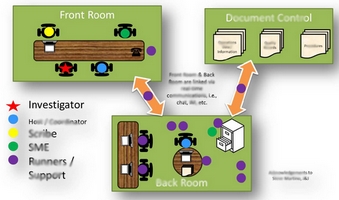
Today, FDA Investigators don’t just pore over your documents, they access your computer-based systems to obtain your information directly! The old way of managing FDA Inspections, while still valid, needs to be updated. This White Paper looks at traditional FDA Inspection approaches, the new “live review” approach, impacts from the “live review” approach, and how to best adapt to the “live review” approach.
Quality Management in the Third-Party Supply Chain
This White Paper provides an in-depth review of contemporary capabilities and solutions for effectively integrating third-party CMOs and other suppliers with GMP manufacturing quality systems and operations. It explores problems with quality oversight in the third party supply chain; how to think differently about managing the third-party supply chain; considering a technology framework to support improved third-party supply chain effectiveness; and how to “make it happen” for your company. This White Paper will stimulate your thinking and engage readers with questions about changing the game with people-process-technology third party supply chain approaches.
White Paper: Quality System Design for Cosmetic Manufacturing

FDA’s June 2013 Guidance for Industry, Cosmetic Good Manufacturing Practices, has outlined FDA’s thinking and expectations surrounding the quality and control approaches to the manufacture of cosmetic products for the US Market. The Guidance incorporates principles of ISO 22716 as part of the international harmonization effort with the International Cooperation on Cosmetic Regulations and: A Business Quality […]

