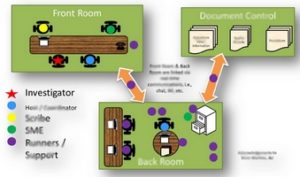
 Today, FDA Investigators don’t just pore over your documents, they access your computer-based systems to obtain your information directly! The old way of managing FDA Inspections, while still valid, needs to be updated. This White Paper looks at traditional FDA Inspection approaches, the new “live review” approach, impacts from the “live review” approach, and how to best adapt to the “live review” approach. Anyone involved in FDA inspections needs to read and understand this material, and determine how best to be prepared for this within their organization.
Today, FDA Investigators don’t just pore over your documents, they access your computer-based systems to obtain your information directly! The old way of managing FDA Inspections, while still valid, needs to be updated. This White Paper looks at traditional FDA Inspection approaches, the new “live review” approach, impacts from the “live review” approach, and how to best adapt to the “live review” approach. Anyone involved in FDA inspections needs to read and understand this material, and determine how best to be prepared for this within their organization.
