Writing for Compliance® now provides more examples, Warning Letter references, exercises, and structure for achieving best-in-class compliance-implicated documents. Incorporating extensive input from former FDA Investigators and feedback from prior participants, the program features more exercises, more assistance with “what does good look like,” and a new, defined writing process to increase consistency and improve compliance writing activities’ outcomes.
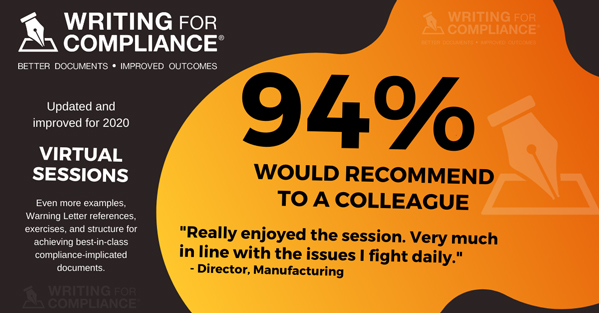
In over seven years of delivering the program to FDA-regulated companies, and with over two hundred mid-to-senior level management personnel having attended, an astonishing 95% stated that the workshop was relevant to the work they currently do, and 94% said they would recommend the workshop to a colleague!