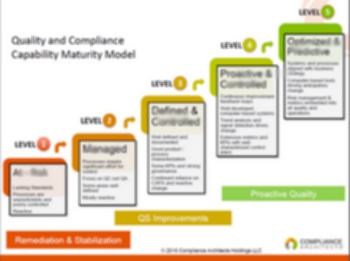
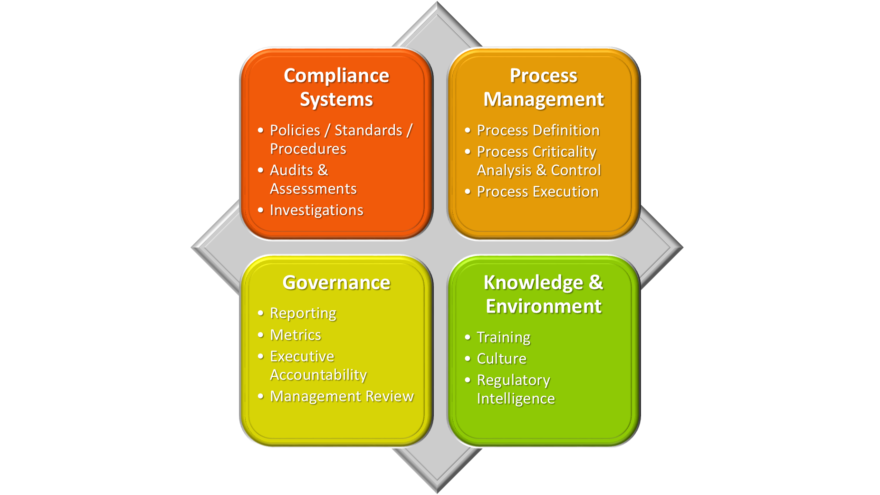
6 Reasons to Work with an Interim Expert/Executive Placement Firm — Amazing How Executives Don’t Recognize the Risks in No. 4
1. The Opportunity Cost of Swapping Staff from One Project to Another:
The need to constantly swap internal resources to keep projects on-track can severely limit FDA-regulated companies from maintaining quality and staying in compliance. Instead of improving operations, preparing for upcoming inspections, optimizing your QMS, keeping that application filing on schedule, or otherwise performing at your peak, you’re wasting time looking for people to keep the company moving forward. Have you considered the cost of missed opportunities for improvement? Time is money, remember that yours is valuable.
2. The Role is New or Needs to be Rebuilt:
New roles are particularly difficult to staff because, typically, there is no benchmark to serve as a point of reference when determining the skills and temperament needed. This is also true for roles in which the predecessor was unsuccessful, and the role will need to be redefined or the department rebuilt. Utilizing extensively vetted interim experts and executives brings proven experience and allows roles and departments to get started on a solid footing before being turned over to salaried staff.
3. Internal Resources are Limited:
Very few companies have the luxury of increasing salaried headcount easily. With a finite amount of money, time and resources, regulatory, quality and compliance departments are consistently stretched too thin. Within today’s drug, device and biotech companies, there’s frequent debates about resources and how they must be ruthlessly allocated. Compliance Architects® has already developed a myriad of tools and strategies to find the perfect interim placement and get them quickly solving your most important challenges.
4. Confidentiality:
When part of your operations is struggling, or you’ve just got a huge backlog of important work, letting the world (or the FDA) know about it isn’t good for business. Advice: Stop posting interim (or permanent) staffing needs on LinkedIn. Privately reaching out to an interim expert and executive placement firm is the obvious solution. Speaking directly to Compliance Architects® is always confidential and speeds up the process of getting the right person working to solve your problems without anyone else knowing a thing.
5. The Role is Beyond Internal Expertise:
Often expert and executive roles are the toughest to staff for since there may not be an internal team member who fully understands and can step-up on a project. Because the skill set is outside the available internal team’s knowledge base, what tends to happen is an unqualified candidate is thrown in and the project suffers.
6. One Bad Hire = Years of Pain:
Logically, the negative impact of a poor hire only increases with the level of influence a position has within a company. Successfully finding a leader who will define strategy, work with other departments, and create processes which will affect future decisions is something that should be carefully considered. The cost of a bad hire in this situation can be astronomical and take years to recover from. Using interim expert and executive placement services can significantly reduce corporate risks while the search for the right candidate takes place.